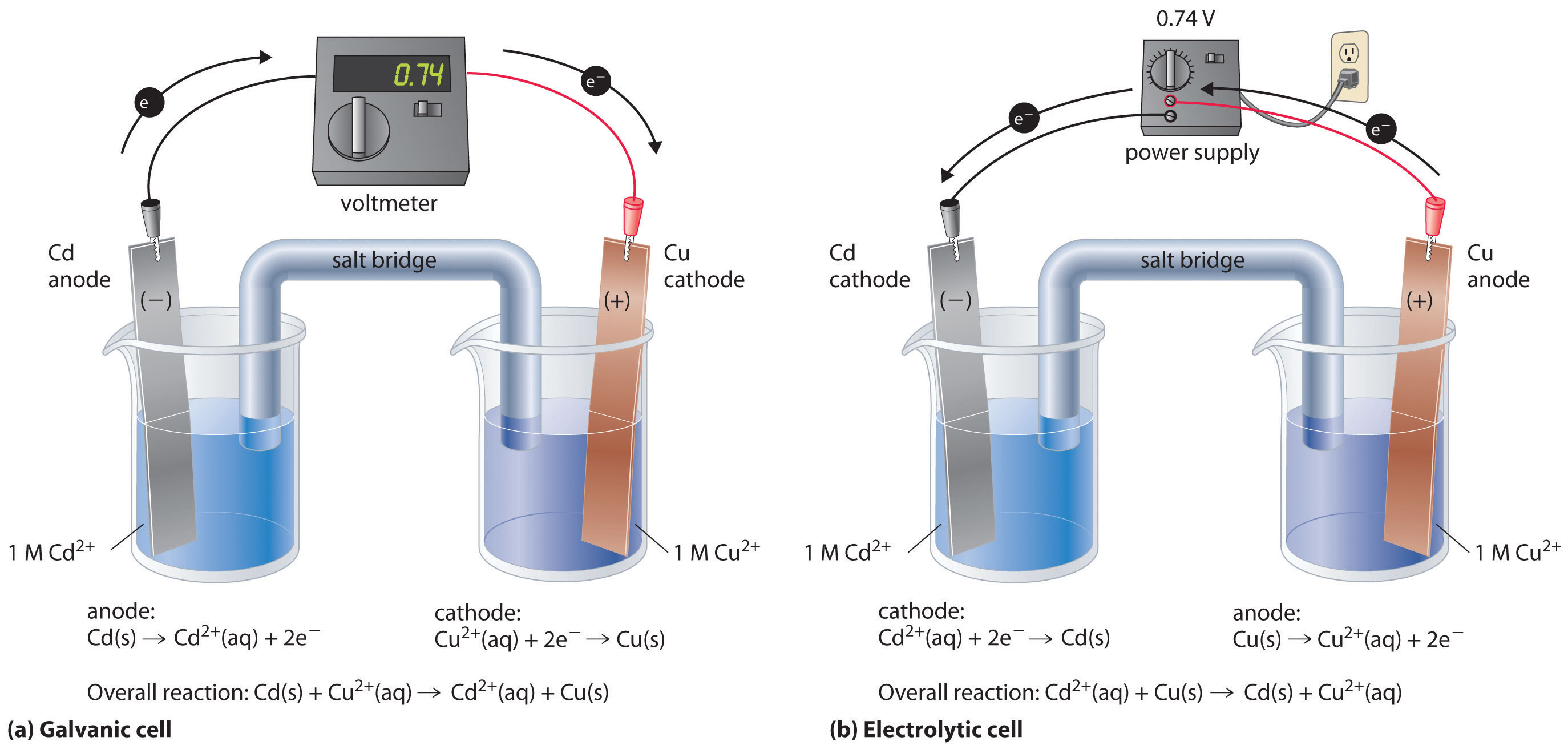
Electrodes In Electrolysis Graphite . however, to obtain graphite electrodes with higher performance, it is essential to deeply understand the. a typical electrolytic cell can be made as shown in figure \(\pageindex{1}\). simultaneously, the understanding of graphite electrode function is continuously expanding, and new strategies for rationally improving performance are being explored. graphite electrodes serve to transfer the electrical energy from the power supply to the steel melt in the eaf bath. graphite electrodes offer remarkable electrochemical properties, emerging as a viable alternative to glassy. besides glassy carbon electrodes, highly ordered pyrolytic graphite (hopg) is commonly used in electrochemistry. Electrodes because they do not take part in the electrolysis reactions. this research introduces a novel, economical approach using graphite felt as a versatile electrode. graphite electrodes are inert close inert unreactive. A method to enhance the typically low.
from usermanualtractors.z1.web.core.windows.net
besides glassy carbon electrodes, highly ordered pyrolytic graphite (hopg) is commonly used in electrochemistry. A method to enhance the typically low. this research introduces a novel, economical approach using graphite felt as a versatile electrode. a typical electrolytic cell can be made as shown in figure \(\pageindex{1}\). however, to obtain graphite electrodes with higher performance, it is essential to deeply understand the. simultaneously, the understanding of graphite electrode function is continuously expanding, and new strategies for rationally improving performance are being explored. graphite electrodes serve to transfer the electrical energy from the power supply to the steel melt in the eaf bath. Electrodes because they do not take part in the electrolysis reactions. graphite electrodes are inert close inert unreactive. graphite electrodes offer remarkable electrochemical properties, emerging as a viable alternative to glassy.
What Happens At The Cathode In Electrolysis
Electrodes In Electrolysis Graphite a typical electrolytic cell can be made as shown in figure \(\pageindex{1}\). besides glassy carbon electrodes, highly ordered pyrolytic graphite (hopg) is commonly used in electrochemistry. graphite electrodes are inert close inert unreactive. Electrodes because they do not take part in the electrolysis reactions. A method to enhance the typically low. however, to obtain graphite electrodes with higher performance, it is essential to deeply understand the. graphite electrodes serve to transfer the electrical energy from the power supply to the steel melt in the eaf bath. graphite electrodes offer remarkable electrochemical properties, emerging as a viable alternative to glassy. a typical electrolytic cell can be made as shown in figure \(\pageindex{1}\). this research introduces a novel, economical approach using graphite felt as a versatile electrode. simultaneously, the understanding of graphite electrode function is continuously expanding, and new strategies for rationally improving performance are being explored.
From dxoargcof.blob.core.windows.net
Electrodes Electrolysis Experiment at Cindy Hopson blog Electrodes In Electrolysis Graphite graphite electrodes offer remarkable electrochemical properties, emerging as a viable alternative to glassy. simultaneously, the understanding of graphite electrode function is continuously expanding, and new strategies for rationally improving performance are being explored. A method to enhance the typically low. however, to obtain graphite electrodes with higher performance, it is essential to deeply understand the. graphite. Electrodes In Electrolysis Graphite.
From www.mbrashem.com
Why Are Graphite Electrodes Used in Electrolysis? M. Brashem, Inc Electrodes In Electrolysis Graphite graphite electrodes offer remarkable electrochemical properties, emerging as a viable alternative to glassy. Electrodes because they do not take part in the electrolysis reactions. graphite electrodes serve to transfer the electrical energy from the power supply to the steel melt in the eaf bath. besides glassy carbon electrodes, highly ordered pyrolytic graphite (hopg) is commonly used in. Electrodes In Electrolysis Graphite.
From www.hhgraphite.com
Graphite Electrodes For Electrolysis Buy Graphite Electrodes For Electrodes In Electrolysis Graphite graphite electrodes offer remarkable electrochemical properties, emerging as a viable alternative to glassy. Electrodes because they do not take part in the electrolysis reactions. graphite electrodes serve to transfer the electrical energy from the power supply to the steel melt in the eaf bath. graphite electrodes are inert close inert unreactive. A method to enhance the typically. Electrodes In Electrolysis Graphite.
From www.carbongraphitematerials.com
graphite electrodes in electrolysis Graphite Electrode Electrodes In Electrolysis Graphite however, to obtain graphite electrodes with higher performance, it is essential to deeply understand the. graphite electrodes are inert close inert unreactive. A method to enhance the typically low. besides glassy carbon electrodes, highly ordered pyrolytic graphite (hopg) is commonly used in electrochemistry. Electrodes because they do not take part in the electrolysis reactions. this research. Electrodes In Electrolysis Graphite.
From www.researchgate.net
Anodic deposition of Au on a graphite electrode. (a) Electrolysis Electrodes In Electrolysis Graphite Electrodes because they do not take part in the electrolysis reactions. A method to enhance the typically low. this research introduces a novel, economical approach using graphite felt as a versatile electrode. graphite electrodes offer remarkable electrochemical properties, emerging as a viable alternative to glassy. graphite electrodes are inert close inert unreactive. besides glassy carbon electrodes,. Electrodes In Electrolysis Graphite.
From labsyst.com
Graphite Rod Electrode Graphite Sheet Counter Electrode Graphite Disc Electrodes In Electrolysis Graphite A method to enhance the typically low. Electrodes because they do not take part in the electrolysis reactions. graphite electrodes are inert close inert unreactive. this research introduces a novel, economical approach using graphite felt as a versatile electrode. graphite electrodes serve to transfer the electrical energy from the power supply to the steel melt in the. Electrodes In Electrolysis Graphite.
From www.carbongraphitematerials.com
graphite electrodes for electrolysis Graphite Electrode Electrodes In Electrolysis Graphite A method to enhance the typically low. a typical electrolytic cell can be made as shown in figure \(\pageindex{1}\). graphite electrodes offer remarkable electrochemical properties, emerging as a viable alternative to glassy. Electrodes because they do not take part in the electrolysis reactions. besides glassy carbon electrodes, highly ordered pyrolytic graphite (hopg) is commonly used in electrochemistry.. Electrodes In Electrolysis Graphite.
From usermanualtractors.z1.web.core.windows.net
What Happens At The Cathode In Electrolysis Electrodes In Electrolysis Graphite besides glassy carbon electrodes, highly ordered pyrolytic graphite (hopg) is commonly used in electrochemistry. a typical electrolytic cell can be made as shown in figure \(\pageindex{1}\). this research introduces a novel, economical approach using graphite felt as a versatile electrode. Electrodes because they do not take part in the electrolysis reactions. A method to enhance the typically. Electrodes In Electrolysis Graphite.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk Electrodes In Electrolysis Graphite graphite electrodes offer remarkable electrochemical properties, emerging as a viable alternative to glassy. besides glassy carbon electrodes, highly ordered pyrolytic graphite (hopg) is commonly used in electrochemistry. this research introduces a novel, economical approach using graphite felt as a versatile electrode. Electrodes because they do not take part in the electrolysis reactions. A method to enhance the. Electrodes In Electrolysis Graphite.
From f69fe4bdad5cc81f.en.made-in-china.com
Graphite Electrode for Electrolysis as Graphite Anodes Rod and Plate Electrodes In Electrolysis Graphite a typical electrolytic cell can be made as shown in figure \(\pageindex{1}\). this research introduces a novel, economical approach using graphite felt as a versatile electrode. graphite electrodes offer remarkable electrochemical properties, emerging as a viable alternative to glassy. graphite electrodes are inert close inert unreactive. besides glassy carbon electrodes, highly ordered pyrolytic graphite (hopg). Electrodes In Electrolysis Graphite.
From www.youtube.com
Physics Experiment Electrolysis Of Water With Aluminium Graphite Electrodes In Electrolysis Graphite simultaneously, the understanding of graphite electrode function is continuously expanding, and new strategies for rationally improving performance are being explored. Electrodes because they do not take part in the electrolysis reactions. besides glassy carbon electrodes, highly ordered pyrolytic graphite (hopg) is commonly used in electrochemistry. graphite electrodes are inert close inert unreactive. graphite electrodes serve to. Electrodes In Electrolysis Graphite.
From www.carbongraphitematerials.com
graphite electrode electrolysis Graphite Electrode Electrodes In Electrolysis Graphite A method to enhance the typically low. besides glassy carbon electrodes, highly ordered pyrolytic graphite (hopg) is commonly used in electrochemistry. graphite electrodes serve to transfer the electrical energy from the power supply to the steel melt in the eaf bath. simultaneously, the understanding of graphite electrode function is continuously expanding, and new strategies for rationally improving. Electrodes In Electrolysis Graphite.
From helpiks.org
ELECTROLYSIS OF AQUEOUSSOLUTIONS Electrodes In Electrolysis Graphite graphite electrodes serve to transfer the electrical energy from the power supply to the steel melt in the eaf bath. besides glassy carbon electrodes, highly ordered pyrolytic graphite (hopg) is commonly used in electrochemistry. a typical electrolytic cell can be made as shown in figure \(\pageindex{1}\). this research introduces a novel, economical approach using graphite felt. Electrodes In Electrolysis Graphite.
From jinsuncarbon.com
Why are graphite electrodes used in electrolysis? Electrodes In Electrolysis Graphite however, to obtain graphite electrodes with higher performance, it is essential to deeply understand the. graphite electrodes are inert close inert unreactive. this research introduces a novel, economical approach using graphite felt as a versatile electrode. graphite electrodes serve to transfer the electrical energy from the power supply to the steel melt in the eaf bath.. Electrodes In Electrolysis Graphite.
From icsechemistry16.blogspot.com
Electrolysis of Copper sulphate using inert electrodes Electrodes In Electrolysis Graphite Electrodes because they do not take part in the electrolysis reactions. graphite electrodes offer remarkable electrochemical properties, emerging as a viable alternative to glassy. graphite electrodes are inert close inert unreactive. graphite electrodes serve to transfer the electrical energy from the power supply to the steel melt in the eaf bath. simultaneously, the understanding of graphite. Electrodes In Electrolysis Graphite.
From www.carbongraphitematerials.com
graphite electrodes electrolysis Graphite Electrode Electrodes In Electrolysis Graphite however, to obtain graphite electrodes with higher performance, it is essential to deeply understand the. simultaneously, the understanding of graphite electrode function is continuously expanding, and new strategies for rationally improving performance are being explored. A method to enhance the typically low. graphite electrodes offer remarkable electrochemical properties, emerging as a viable alternative to glassy. Electrodes because. Electrodes In Electrolysis Graphite.
From www.hhgraphite.com
Graphite Electrodes For Electrolysis Buy Graphite Electrodes For Electrodes In Electrolysis Graphite Electrodes because they do not take part in the electrolysis reactions. besides glassy carbon electrodes, highly ordered pyrolytic graphite (hopg) is commonly used in electrochemistry. A method to enhance the typically low. graphite electrodes offer remarkable electrochemical properties, emerging as a viable alternative to glassy. a typical electrolytic cell can be made as shown in figure \(\pageindex{1}\).. Electrodes In Electrolysis Graphite.
From hbhongkuo.en.made-in-china.com
Graphite Electrodes Electrolysis UHP HP RP Electrode for Electric Arc Electrodes In Electrolysis Graphite graphite electrodes serve to transfer the electrical energy from the power supply to the steel melt in the eaf bath. simultaneously, the understanding of graphite electrode function is continuously expanding, and new strategies for rationally improving performance are being explored. a typical electrolytic cell can be made as shown in figure \(\pageindex{1}\). A method to enhance the. Electrodes In Electrolysis Graphite.